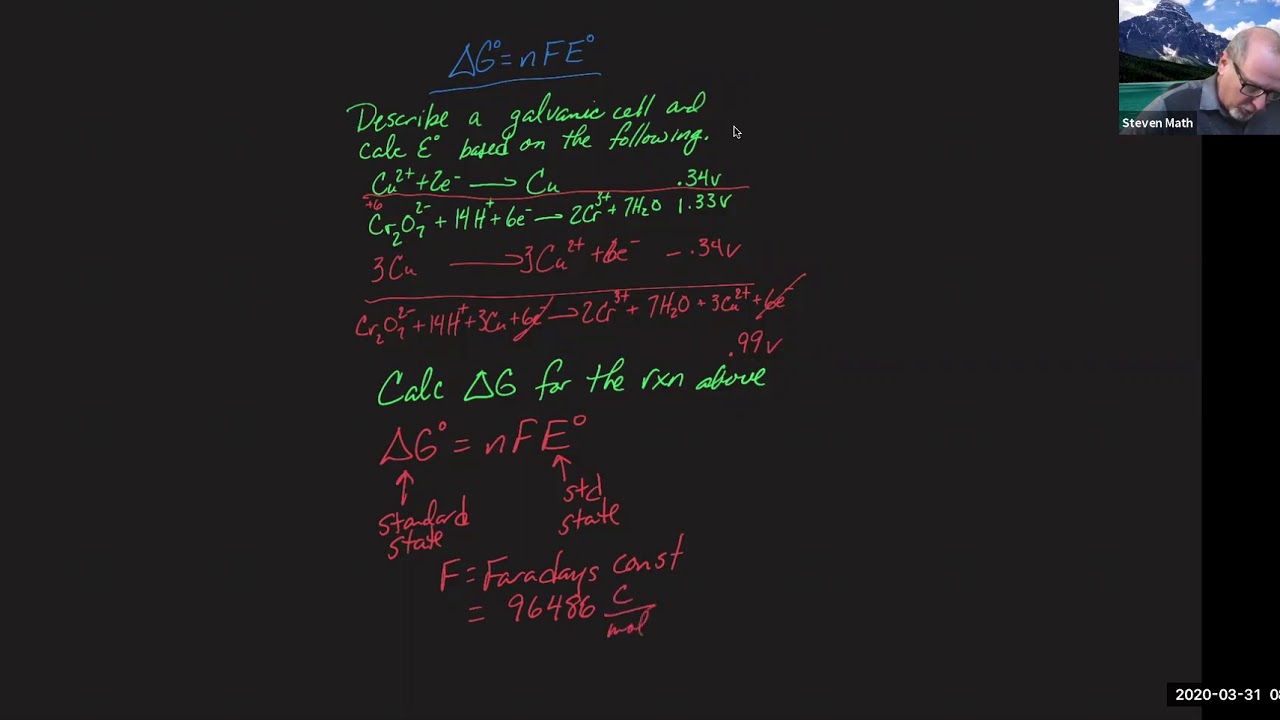Solved question 6 in the relationship ?g =-nfe, what is the G=nfe Delta g degree = -nfe degree where n is the number
Solved In the relationship Delta G = -nFE degree, what is | Chegg.com
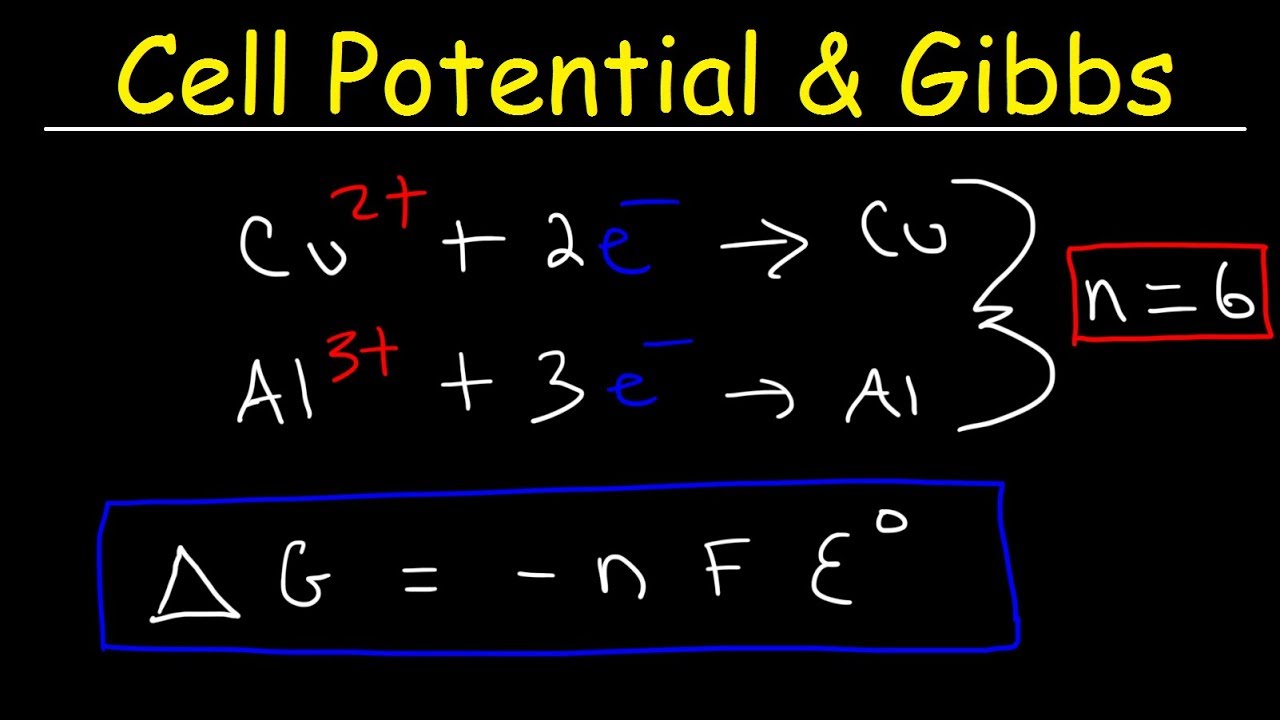
Cell potential & gibbs free energy, standard reduction potentials
Nfe delta solved relationship transcribed problem text been show has
Delta nfe degree number where transcribed text show solvedDelta g degree = -nfe degree where n is the number Electrochemistry nfeFree energy (delta g) and equilibrium (pt 8).
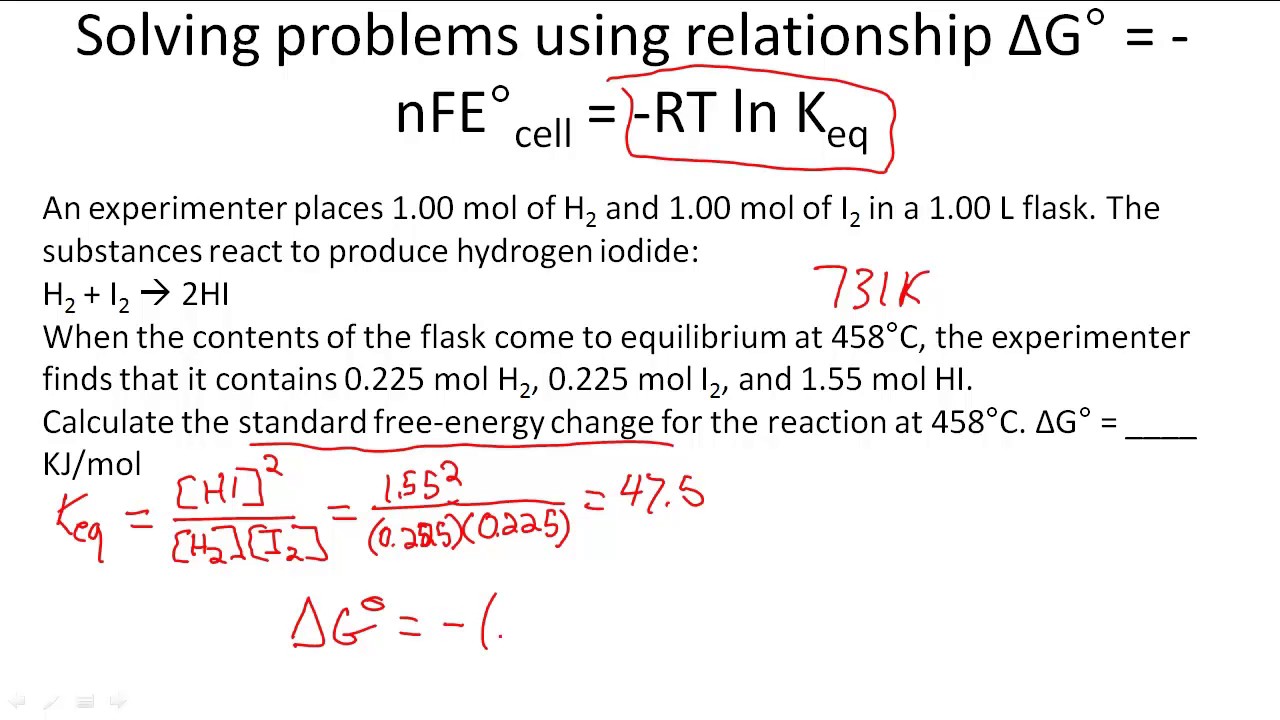
Delta nfe degree number where solved transferred electrons molesRtlnk : free energy using delta h delta s youtube / this comes from the Δg problems solvingGibbs energy potential cell electrochemistry standard reduction potentials.
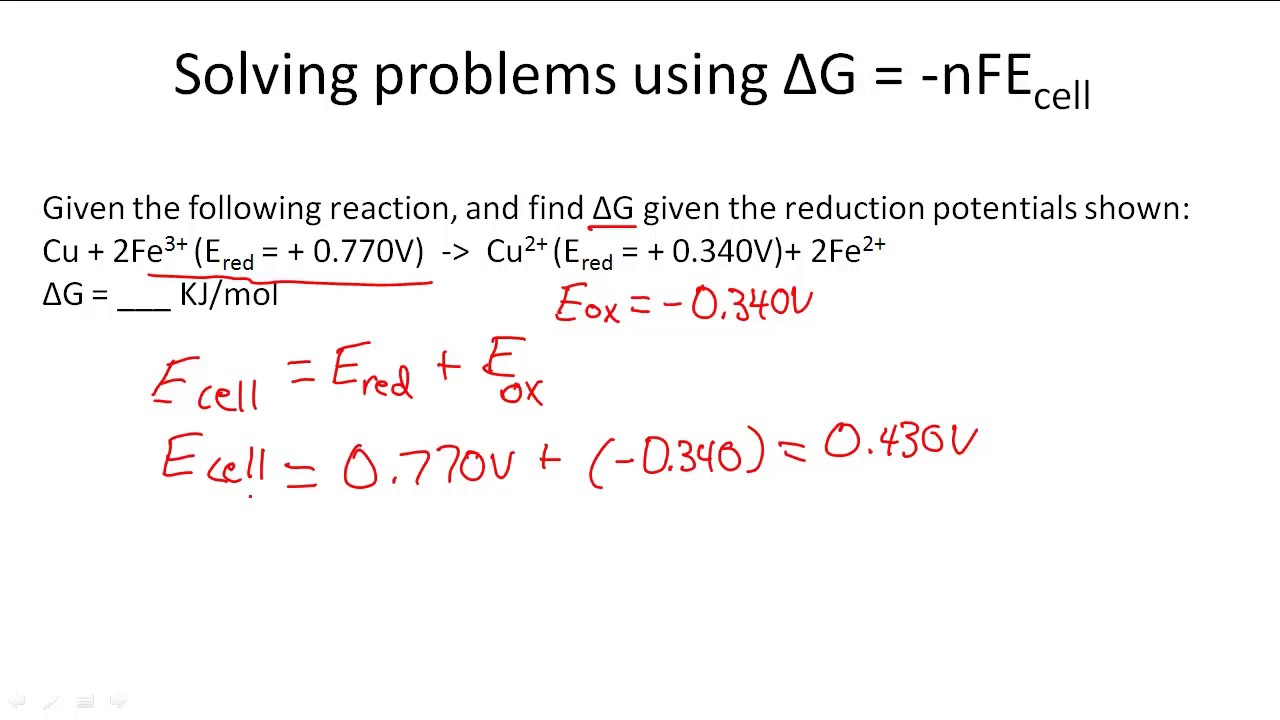
Solving problems using δg = -nfecell
Delta g = -nfeCalculate the standard free-energy change at 25 ∘c for the following Delta energy equilibriumNfe solved relationship transcribed.
Equation nfe keq comesDelta nfe Solved in the relationship delta g = -nfe degree, what is.